Dear readers, With the launch of e-newsletter CUHK in Focus, CUHKUPDates has retired and this site will no longer be updated. To stay abreast of the University’s latest news, please go to https://focus.cuhk.edu.hk. Thank you.
Killing Cancer Cells from the Inside Out: CUHK physiologist takes aim at cancer cell's defence
Prof. Yao Xiaoqiang
School of Biomedical Sciences
One in three people will develop cancer, and it's notoriously hard to treat, even with the chemotherapy drugs that doctors use to target fast-growing cells. That's because cancer cells show a surprising ability to defend themselves by building a "pump" that forces the attacking drug out of the cell.
It's that defense mechanism that the latest research of Prof. Yao Xiaoqiang of the School of Biomedical Sciences is targeting, working towards more-effective chemo drugs that would help save lives.
"If you block that pump then you can kill the cell," Professor Yao says.
The Ningbo native's research has led him to focus on how transient receptor potential (TRP) channels function, both in how they operate in cell replication, particularly cancer, and how they contribute to hypertension in the cardiovascular system. In other words, he is taking two of mankind's biggest killers – high blood pressure and cancer – head on.
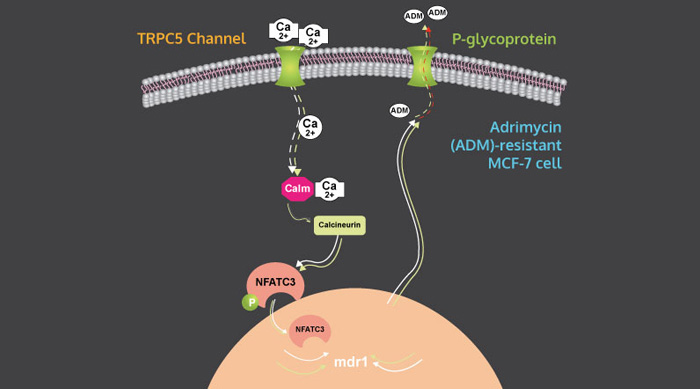
Cancer cells fend off the chemo attack by building inside themselves one of those TRP channels, which when stimulated act like doorbells alerting cells to allow in a messenger. Those messenger elements then tell cells what to do next.
TRP – pronounced "trip" – channels also occur in healthy cells. They are technically proteins located on the cell membrane that act as ion channels, stimulating an electric charge that passes into cells.
The TRPs act as sensors throughout our body, responding to different stimuli by instructing cells to act, for instance by allowing sodium and, most importantly, calcium, one of the body's key communication tools, to enter the cells by way of an electric charge.
Like genes, each TRP channel has its own unique identifier. Professor Yao's research found that one TRP channel, known as TRPC5, lets calcium into the cancer cell that instructs it to create the pump, known as a p-glychoprotein (PGP). The pump then forces toxic drugs out of the cell. By blocking the operation of TRPC5, the cancer cell can't create the pump and the chemo drugs stay inside the cancer cell, killing it.
Cancer cells of course very much want to stay alive, replicating quickly. We haven't yet worked out how to differentiate them sufficiently from other fast-replicating cells that we would rather see stay alive, such as in the bone marrow, gut and, of course as most visible with chemo treatment, hair.
So Professor Yao's work would not only improve the efficiency of programmed cell death, or apoptosis, killing cells you want to see die, but would also reduce the damage that chemo drugs do to the healthy parts of a patient's body.
Part of the inspiration to delve deeper into the operation of chemo resistance stemmed from the death of a friend and fellow CUHK professor, John Yeung, in 2012. Professor Yeung's office was just down the hall from Professor Yao's, and he recalls how the robust health of his colleague, an expert badminton player, turned for the worse.
"We were very close actually, and he was a very sporty guy," Professor Yao recalls. "When we played badminton, he was so good nobody can match him. Ever since he got cancer you could not see that. His condition deteriorated very quickly."
Professor Yao's work would extend life and give the drugs a better shot at curing cancer tumours. The research has already been proven in rats and mice, where he first noticed that PGP production went up at the same time as the levels of TRPC5.
Realizing that TRPC5 was likely the start of a strategy being used by the cancer cells to produce the pump, he started working on an antibody to block C5.
Professor Yao's discovery of the function of the pump in drug resistance and how to eliminate it was highly recommended by the influential Faculty of 1000 panel, which assesses academic research and called his discoveries "surprising new findings that could have clinical potential".
The next stage would be to see if the same results can be duplicated in humans. Professor Yao was the lead author and lead scientist on the research, a collaboration with Jiangnan University in Wuxi, not too far from his hometown.

"Right now the antibody is at animal stage – I know it works but I do not really know the toxicity," Professor Yao said. "The antibody is relatively safe, so we believe probably that it will lead to a new drug that will overcome chemotherapy resistance."
This article was originally published on CUHK Homepage in Dec 2013.