Dear readers, With the launch of e-newsletter CUHK in Focus, CUHKUPDates has retired and this site will no longer be updated. To stay abreast of the University’s latest news, please go to https://focus.cuhk.edu.hk. Thank you.
Chance and Choice in a Chemistry Career
Steve Tse brings chemistry and computing together

Einstein has famously said that God does not play dice. What he refers to is that physical nature follows its own laws and nothing is left to chance. One such rule is chirality in the world of molecular chemistry.
Prof. Steve Tse of the Department of Chemistry at CUHK teaches two undergraduate courses on physical chemistry. He uses the human hands to illustrate chirality. The right and left hands of a human being are mirror images of each other. Yet the two cannot be superimposed on each other. Similarly, many biologically active molecules, including the naturally occurring amino acids (the building blocks of protein) and sugars, are chiral in nature. The molecules share the same physical properties (e.g., boiling point) but their two halves respond differently to light and some biological processes.
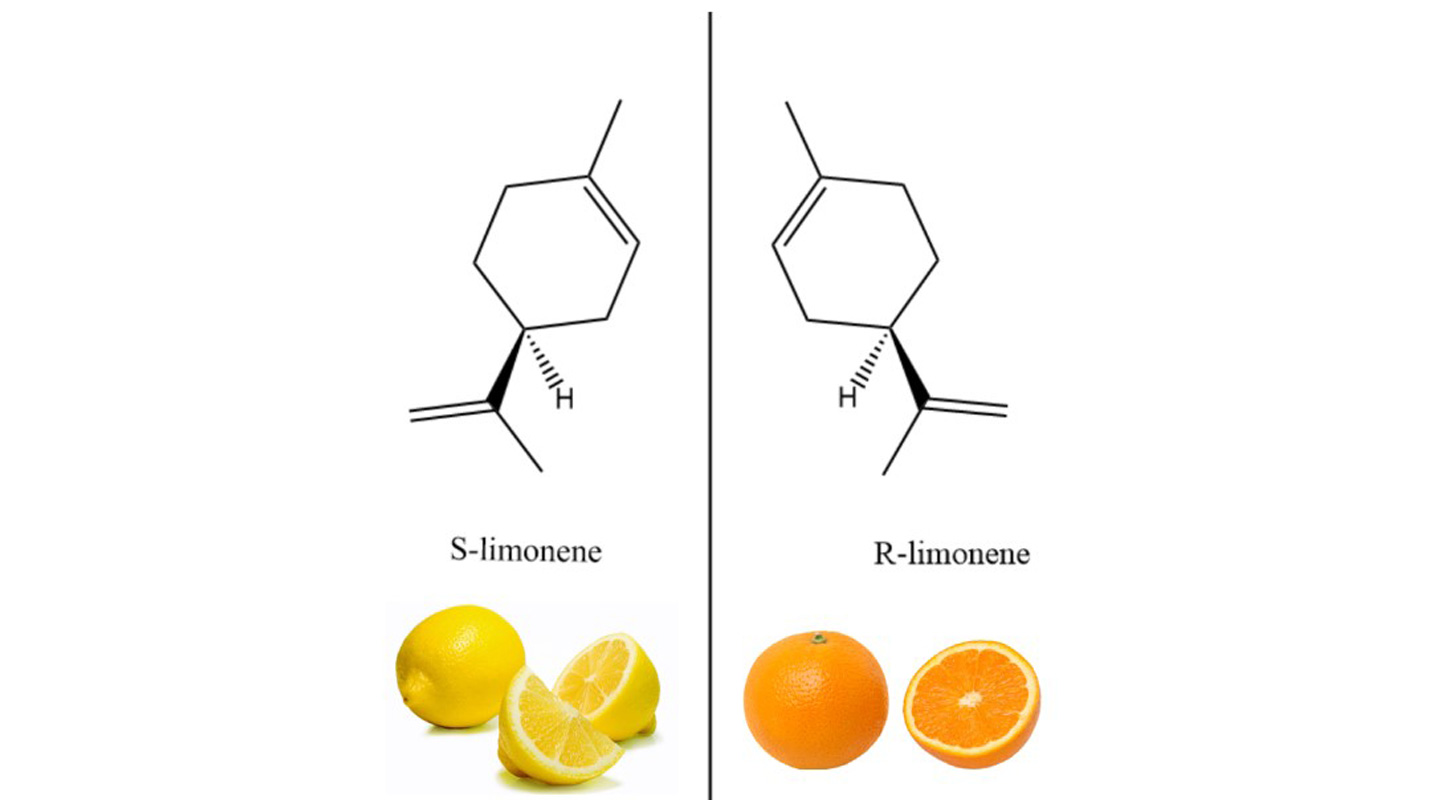
In Figure 1 above, the two compounds are mirror images of each other, with the same atoms and the same angles and distances between atoms. But they cannot be superimposed on each other. The compound on the left, S-limonene, smells like a lemon, whereas the one on the right, R-limonene, smells like an orange.
Professor Tse explained the sensation of smell from a molecular perspective: ‘Smell is due to the response in our brain from a signal sent by biological receptors that have interacted with the chemical. The interaction depends on the chemical nature of the receptor and the chemical.’
Chirality can also be understood with the lock and key metaphor, Professor Tse further explained. It takes the right key to trigger a process to unlock an outcome. The molecules of S-limonene are a key to release on the nerve receptor the signal to the brain that gives rise to the sensation of the smell of lemons. The different key of the R-limonene molecules would open a different lock and lead to an orange smell.
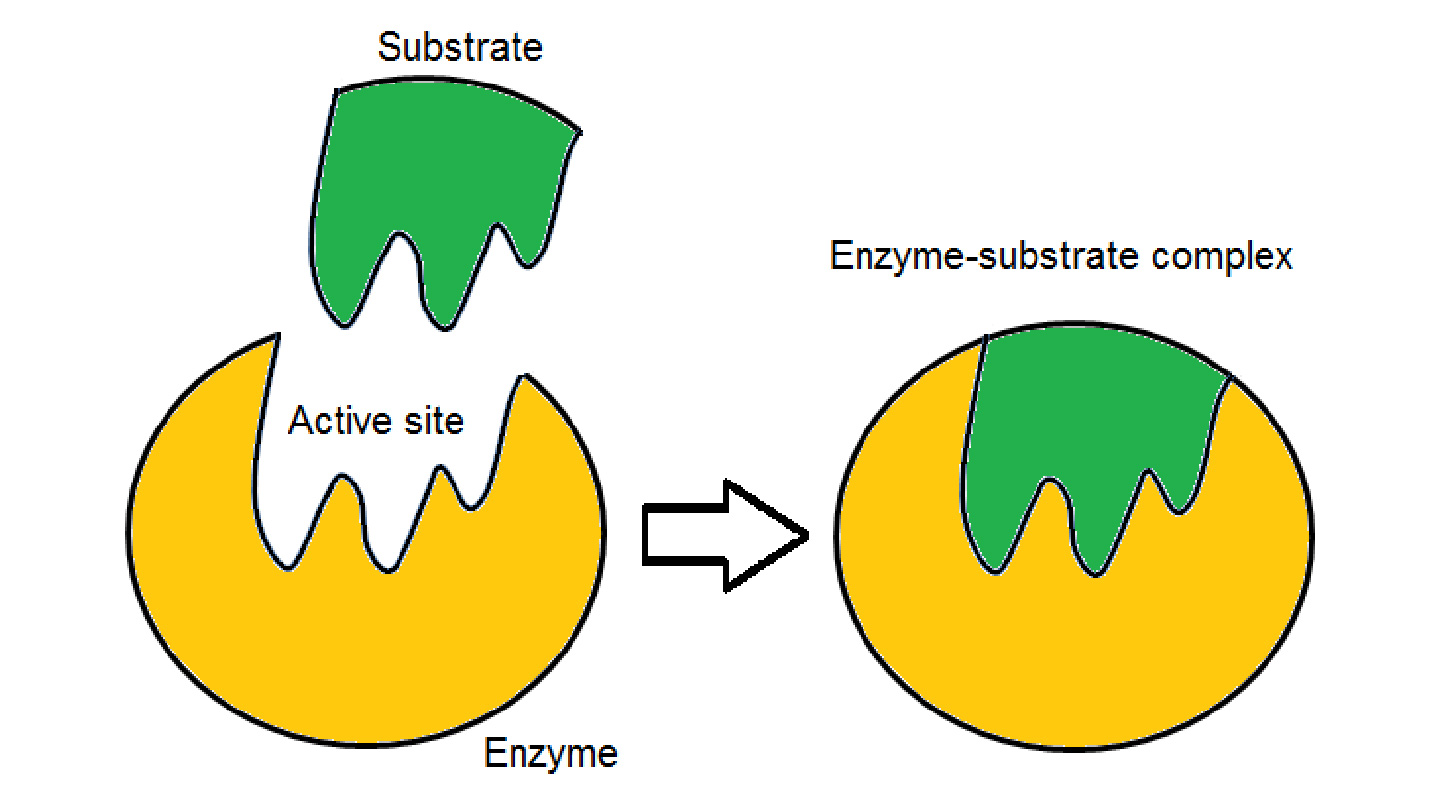
The implications of chemical chirality for the manufacturing of drugs are obvious. To bring about the desired medical outcomes, drugs that need to be chiral to function need to have the right or exact chirality. The wrong type of chirality may lead to no or even harmful effects.
Since only a part or parts of a compound holds the key to eliciting the desired outcome, it is necessary to isolate or separate the relevant part or parts. But in reality such isolation or separation is extremely difficult if not impossible due to the great similarity in chemical structure of the compounds involved. Just imagine the toil of telling one identical twin from another.
The CUHK team of chemists comprising Chairman Prof. Yeung Ying-yeung and Professor Tse took a different approach. They did not play dice but rather tried to build compounds with specific chirality, that is, they aimed at synthesizing only one type, right or left, of the molecule but not the other type or the whole.
The team has devised a catalytic system for the creation of new halo-spiro compounds. Spiro compounds are structures of two molecular rings joined by a common atom.
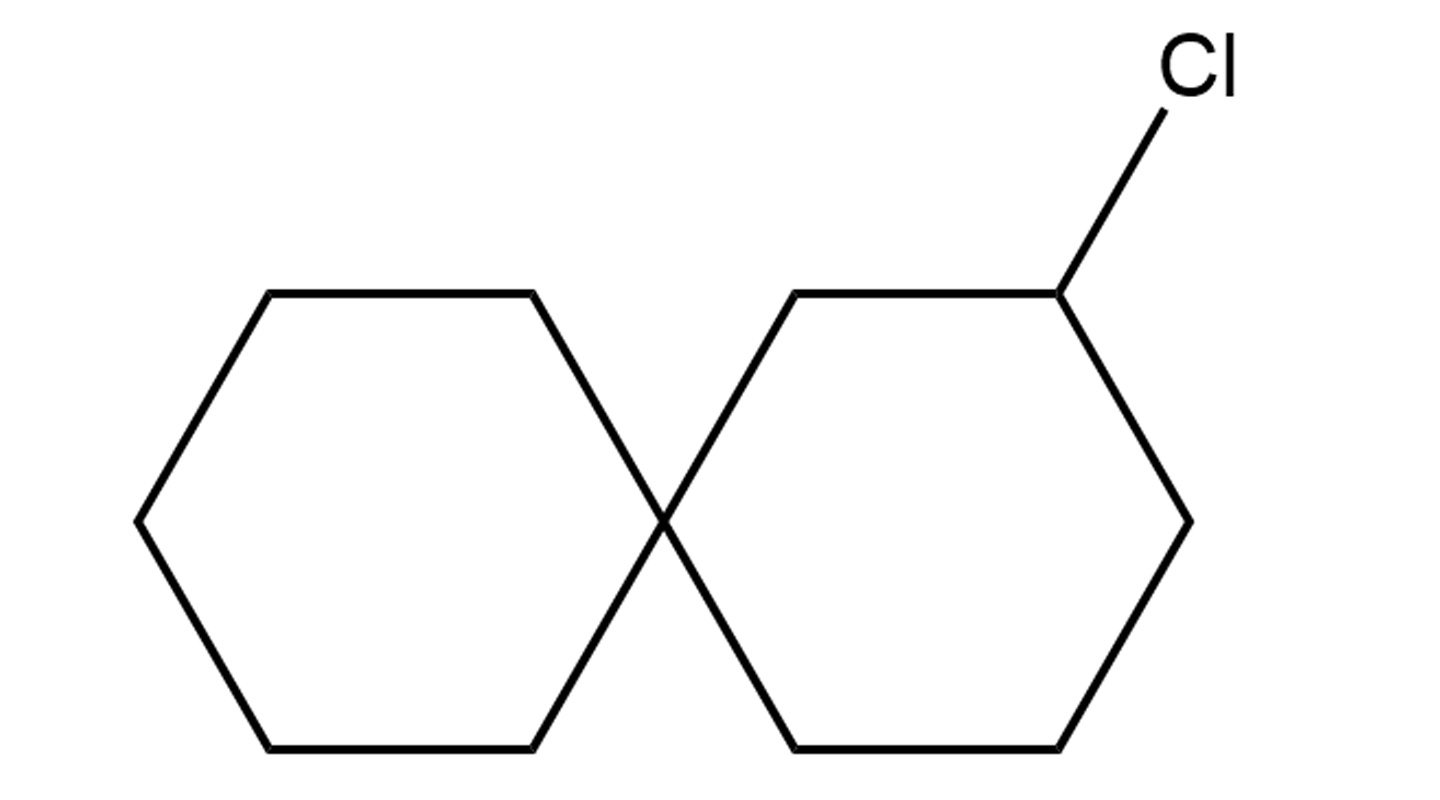
They are the building blocks of larger chemical structures (this way they are like Lego cubes). When a halogen atom (one that belongs to the halogen class that includes the more familiar chlorine, iodine, etc., atoms) is added to a spiro compound, the malleability or highly reactive nature of the former provides the necessary anchor for other units, thereby allowing the chemist to build more elaborate chemical motifs for more complicated chemical structures.
The CUHK team’s halocyclization-based catalytic system is a system in which catalysts are added to enhance the production of chiral halo-spiro compounds. Being able to make spiro rings, add halogen atoms and determine chirality, their method enhances the ability to modify the halo-spiro compounds and greatly enriches the chemical toolkit of the pharmaceutical industry for new drug design options. Their research findings have been published in the top science journal Nature Catalysis.
In developing this novel catalytic system, Professor Tse’s role laid more in the calculating than in the mixing. His training in computer science at Towson University in the US has made his role pivotal in the research. Whether in nature or in the laboratory, the number of molecules to a chemical reaction is astronomical, easily surpassing 100,000,000,000,000,000,000,000. It is well-nigh impossible to take all the atoms and molecules into the calculations. Not all the details are required, anyway. Neither are all the components in a reactant molecule relevant for understanding the experiment.
Professor Tse said, ‘If they are not all relevant, maybe we don’t need to describe them with as much detail or include them at all. The bottom line is we need to have chemical intuition about what details are important for studying the system and focus on only the details that are important and affordable.’
With the sophisticated computing facility at the Department of Chemistry, Professor Tse was able to arrive at numerical approximations for the quantum mechanical calculations that describe the behaviours and reactions of the compounds under study, thereby providing the theoretical bedrock for the novel catalytic system for the preparation of chiral halo-spiro compounds.

This may sound like serendipity or déjà vu in retrospect, but Professor Tse had not played dice to find a specialty for himself. He followed his heart and the self-understanding of what he wanted. But he did have his moments of doubts. He admitted: ‘Just like many science students in Hong Kong, I worried about my future and didn’t choose pure science as my undergraduate major at Towson University. So I majored in computer science. But even as a computer science major, I still took many chemistry classes because I was genuinely interested in the subject.
‘I did quite well in all the chemistry classes and was given many opportunities at the chemistry department. I was eventually convinced to become a chemistry major since I thought following my interest was more important. With my computer science background and chemistry, computational or theoretical chemistry seemed to be the next natural choice for me. That was why I went on to get a PhD in theoretical chemistry at Stanford University and did a theoretical chemistry postdoc at the University of Chicago.’
Since joining CUHK in 2015, Professor Tse has been teaching and supervising undergraduate and postgraduate students. While he has no crystal ball about trends and markets for his students, he is very much concerned with their academic choices and development. He believes that, much as the law of chirality has left very little chance for the molecules’ journey in nature, one can always find good fits for one’s talents and aspirations on one’s intellectual journey.
By tommycho@cuhkcontents